“Why do your products contain these inflammatory oils?” is the type of loaded question I often get “asked”. Despite my endeavours to quash people’s seed oil anxieties, this sort of emotion-based tirade continues to be hurled in my direction. I’ve addressed the misinformation regarding seed oils extensively by way of three articles – The Omega Ratio: Useful Or An Unnecessary Hassle? [1], The Exoneration of Seed Oils: A Review of Misinformation and the Evidence” [2] and The Exoneration of Seed Oils Part 2: A Bayesian View of Seed Oil Consumption and Health [3] – yet there’s still much more that needs to be said on this oddly polarising topic.
One thing in particular that keeps rearing its ugly head is the claim that “seed oils cause inflammation”. Although I’ve previously tried to curb the rhetoric that seed oils are proinflammatory, this fear appears to be a key focus for the anti-seed-oilists. This article will zone in on this aspect of seed-oil negativity in more depth. I’ll look at the biochemical processes, mechanistic data and epidemiology to better demonstrate the fallacy of this claim. Now, I realise my last sentence could reek of confirmation bias and infer that I’m looking only to endorse my prior position, and I could be criticised for not being very scientific. However, as I’ve already extensively reviewed my stance (see Part 2), here I’m merely elaborating on an already stated position, so it’s valid to tackle a specific aspect of the controversy in this manner.
Fatty Acids and the Inflammation Process
Inflammation is a necessary physiological response to injury or infection and is part of the natural healing process. Prolonged low-grade inflammation is the chronic production of inflammatory factors at a low level as a result of an unresolved inflammatory response, and can be involved in the pathogenesis of non-communicable diseases (NCDs) like cardiovascular disease (CVD), some cancers, depression, chronic pain, gut issues and obesity [4]. Poor dietary habits have been shown to be associated with low-grade inflammation [5], and this has led to assertions that the consumption of seed oils is linked to inflammation and therefore increases the risk of associated NCDs [6]. But what’s the rationale behind these claims?
When people talk about seed oils, they typically mean sunflower, rapeseed (also known as canola), safflower, corn and soybean oil. These oils are typically high in omega-6 fatty acids, particularly linoleic acid (LA). LA is one of two essential fatty acids, the other being alpha-linolenic acid (ALA), an omega-3. Omega-3s and -6s are both polyunsaturated fatty acids (PUFAs). Humans require a regular intake of both ALA and LA to prevent illness. In the absence of dietary LA, children will fail to grow and adults may experience fatty liver, skin lesions, reproductive failure and, eventually, death. Fortunately, LA is abundant in most varied diets, whether or not they contain seed oils, and consequently, most Western diets contain ample amounts and deficiency is very rare. Those concerned about seed oils argue that not only do we not require additional LA, but that high intakes are harmful since, they assert, consuming it in excess leads to systemic inflammation. The justification for this comes from speculation regarding the conversion of LA to arachidonic acid (AA). AA is another omega-6 and can be synthesised in vivo from LA, and, in certain circumstances, is considered semi-essential as consuming it directly from food reduces the requirement for dietary LA.
Through several complex biochemical pathways, AA is involved in the production of various inflammation-promoting factors. Oxylipins are chemical messengers that play critical roles in the immune and inflammatory response, the most common of which are eicosanoids. AA, along with other long chain fatty acids, such as the omega-3 fatty acid eicosapentaenoic acid (EPA – found in oily fish and types of algae), is necessary for the production of eicosanoids. When stimulated, both fatty acids are released from cell membranes to be available for eicosanoid production. The response from eicosanoids produced from AA, however, is different to the response from those derived from EPA. The latter are less potent inducers of inflammation [7], and this has led to claims that AA-derived eicosanoids are inflammatory. Those making the claim are assuming that excess dietary LA leads to greater conversion to AA, which, in turn, is converted to inflammatory eicosanoids.
Arachidonic Acid and Inflammation
This explanation of how seed oil consumption contributes to increased inflammation sounds very plausible. However, as always, the reality is way more complex, and it’s a mistake to make this connection for two key reasons. Firstly, excess dietary LA doesn’t necessarily mean more AA is produced. A negative feedback control involving multiple factors is in place, meaning that both AA and eicosanoids are produced only on demand [8]. Secondly, AA-derived eicosanoids both inhibit proinflammatory factors like leukotrienes and cytokines and induce anti-inflammatory agents known as lipoxins [9]. Moreover, oxylipin synthesis relies on different enzymes, and which eicosanoids are produced is determined by the combination of the fatty acid precursor and which enzyme is activated. As well as this, another class of regulators known as specialised pro-resolving mediators (SPMs) are involved. SPMs are derived from both omega-3 and omega-6 fatty acids and function as local mediators, actively turning off the inflammatory response [10], and the resulting SPM is dependent on which enzyme is activated and numerous other stimuli, not just which PUFA is present [11].
Another key blood marker for inflammation, C-reactive protein (CRP), has been shown to be lower when serum LA is higher [12], indicating that a high intake of LA may actually protect against low-grade inflammation. It’s also been demonstrated that even at very high intakes, LA doesn’t increase the inflammatory response, nor does it have a significant impact on AA levels [13]. The claim that a high intake of omega-6 fatty acids, or indeed LA-rich sunflower oil, promotes low-grade inflammation by increasing the body’s AA levels is overly simplified, and in fact, the reverse is more likely [14]. Indeed, it also appears to be the case that adequate AA levels may help to maintain an optimal inflammatory response.
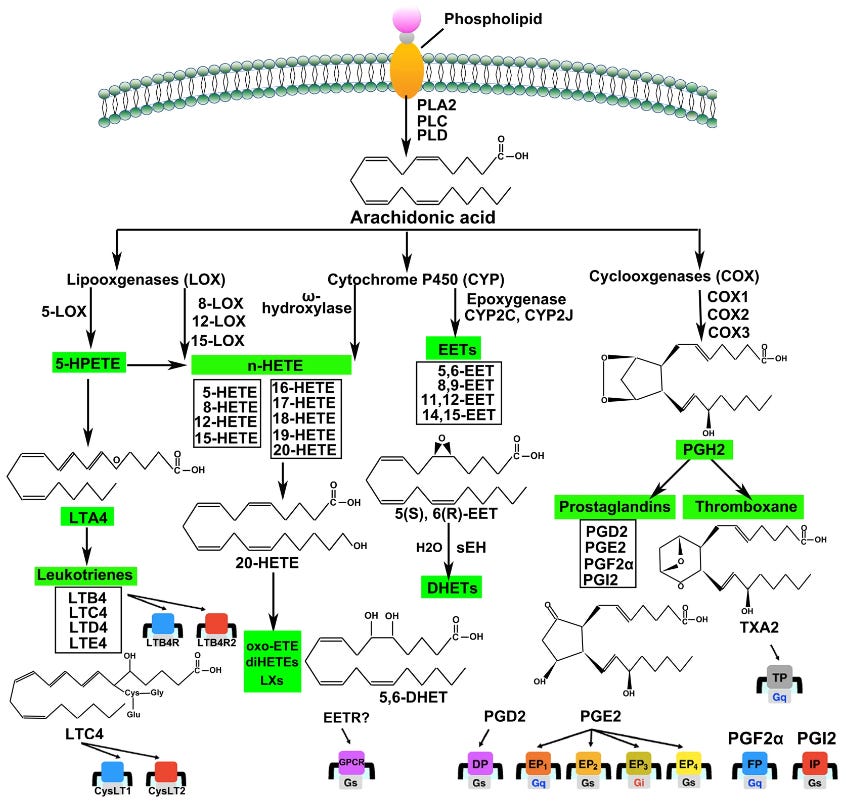
If you found the past three paragraphs hard to understand, that’s because the mechanisms are extremely complicated. The diagram below is a simplified(!) overview of the AA metabolism pathways (from Wang, et al. (2021)) [15]. The details are not relevant: I’ve included it to illustrate that the mechanisms by which long-chain PUFAs, such as AA, are involved in the inflammatory response are immensely complex, involving multiple pathways, numerous enzymes, substrates, hormones and other stimuli, and feedback loops. Simply stating that having an excess intake of LA leads to too much AA and that this causes more inflammatory eicosanoids to be produced is ridiculously simplistic.
The 3 to 6 Ratio and Inflammation
An insufficient intake of omega-3 fatty acids will likely have an adverse effect on inflammatory markers [16], and because omega-3s and -6s compete when it comes to eicosanoid production – due to the fact that they share some of the enzymes involved – some claim that an imbalance in the ratio of omega-3 to -6s may lead to an imbalance in the inflammatory signalling molecules. Indeed, it’s been suggested that the ratio of omega-3 to -6 should be no more than 1:4 (i.e. up to four times more omega-6s than -3s) [17]. Our hunter-gatherer ancestors, depending on where they resided, through acquiring their nutrition from a range of foods, may have had these sorts of intakes. Intakes of many modern diets, however, have led to large amounts of omega-3s being omitted, and omega-6s are more prevalent, leading to typical ratios of 1:15 to 1:20 or even higher [18].
However, as discussed in The Omega Ratio, people wrongly assume that the issue lies on the omega-6 side of the equation, rather than the problem being a lack of omega-3s that contributes to low-grade inflammation. When, as demonstrated by one study, intakes of ALA from flaxseed oil are increased, more EPA is produced and the omega-3 to -6 ratio improves [19]. Neither the 3 to 6 ratio nor the level of omega-6s is the issue, and we should be encouraging strategies that promote more dietary omega-3s rather than fearmongering against omega-6s.
Seed Oils and Inflammation: The Epidemiology
What does the epidemiology more generally tell us about the link between seed oils, LA and inflammation? Fortunately, there’s been a fair bit of research in this area. One study had 13 overweight men fast overnight and then consume one of two muffins: one with 50 g of butter or one containing 50 g of sunflower oil, with all subjects completing both interventions on separate occasions. The results showed that after consuming the butter muffin, the inflammatory biomarker interleukin-6 (IL-6) increased, whereas after consuming the sunflower oil muffin, several inflammatory markers decreased. These findings indicate that sunflower oil reduces inflammation relative to butter [20].
Another trial split overweight subjects into three groups. One group consumed 20 ml of rapeseed oil daily for 12 weeks, another had 20 ml of sunflower oil and the controls had their normal diet. After the 12-week-period, the subjects were tested for various inflammatory biomarkers, all of which showed little change. Moreover, at the outset, butter and olive oil were the most consumed fats, which indicates that when olive oil and butter are replaced with seed oils, there’s no worsening of inflammatory markers [21]. In another study, researchers took 67 overweight people and, for 10 weeks, fed them either a diet high in omega-6-rich vegetable oil or one high in saturated fat from butter. The vegetable oil group lost liver fat and improved metabolic status without losing weight, whereas the butter group had higher liver fat, insulin, cholesterol, LDLs and triglycerides [22]. These results tell us that vegetable oils don’t cause signs of inflammation.
In yet another trial, this time on 486 middle-aged women, the consumption of sunflower, corn, rapeseed, soybean and olive oils was shown to lower levels of several inflammatory biomarkers including CRP and IL-6 [23]. More robustly still, a review of 37 randomised controlled trials looking at dietary fats and inflammatory markers found that, out of 10 controlled trials involving vegetable oils, none demonstrated significantly increased inflammatory markers. In fact, three of them even found possible beneficial effects on inflammation [24]. The claim that a high intake of omega-6 fatty acids or LA-rich seed oils promotes inflammation is unsubstantiated, and it’s more likely that the reverse is true: their consumption offers some protection. In one review, the authors concluded that “virtually no evidence is available from randomised controlled intervention studies among healthy [people] to show that addition of LA to the diet increases the concentration of inflammatory markers” [25].
Arachidonic Acid: In Vivo vs. Dietary
Most of the claims that seed oils cause inflammation relate to an excess consumption of LA being converted to AA, which, as you now know, doesn’t hold up. The conversion of LA to AA is a three-stage process beginning with LA being converted to gamma-linolenic acid (GLA), a fatty acid that’s also consumed in its preformed state from the consumption of certain plants. GLA is then converted to dihomo-gamma-linolenic acid (DGLA), which is subsequently converted to AA [26]. Due to there being three stages, inevitably, efficiency of conversion is reduced to as little as one to 10 percent [27]*, depending on numerous factors such as sex and genetic make-up [29]. However, not only does the research tell us that increased dietary LA does not increase eicosanoid production, but also that consuming more dietary LA doesn’t increase tissue levels of AA much; in fact, there may even be an inverse relationship [30]. A 2011 systematic review of human trials of LA consumption and subsequent changes in tissue levels of AA found that modifying dietary LA intake has little effect on blood AA levels in adults consuming Western-type diets [31]. Of the 16 papers reviewed, only one reported a significant increase in AA content (from an 86 percent increase in LA intake) [32]. Indeed, three of the studies reported reductions following increases in LA consumption [33].
But what about preformed AA consumed directly from the diet? Is this harmful? The principal dietary sources of AA are eggs, meat and fish, and typical intakes are estimated to range from 50 to 300 mg/d for adults consuming Western-style diets [34]. While the claims that very high levels of AA – whether acquired in the preformed state or from LA conversion – are linked to the production of eicosanoids and disordered platelet aggregation [35], research has indicated that consuming dietary AA at levels of up to 1.5 g per day is harmless in healthy adults [36]. This is good news for carnivore and paleo diet proponents who can breathe a sigh of relief knowing that their high consumption of preformed AA will have no adverse effect on their health. But how do their blood levels compare to AA converted from LA in vivo? Let’s compare the intake of someone following a typical Western diet to someone following an extreme carnivore regimen. A typical Western dieter consuming 2,500 calories of food per day would have 30 percent of those calories derived from animals [37] – i.e. 750 calories’ worth. Assuming such a diet supplies the upper end of the typical AA intake range, this might equate to 300 mg per day. Compare this to a 2,500-calorie-per-day carnivore diet, 95 percent of whose energy comes from meat, fish, milk and eggs. This individual could, therefore, be consuming as much as 950 mg of AA per day: well below the safe 1.5 g per day. Phew!
But what about someone who includes seed oils in their diet? Typical intakes of LA are from 12-13 g to 17-20 g per day for women and men, respectively [38]. Assuming an average efficiency of conversion (five percent), up to 1,000 mg of AA could be produced.
Accepting that, despite being pulled from the literature, these figures are, nevertheless, imperfect and based on assumptions, what am I trying to illustrate, especially as I risk losing the reader to excessive nuance? I’m showing that there’s a fundamental point to be made. My review of the biochemical, mechanistic and epidemiological data runs contrary to the claims that seed oil consumption leads to increased inflammation, clearly showing the assertion to be baseless. However, even if the claim that seed oil consumption causes inflammation through an increased LA intake leading to greater in vivo AA production were to be true, and that this, in turn, leads to greater inflammatory eicosanoid synthesis (which it doesn’t), the AA blood levels of meat-heavy diets will likely be similar to those who consume typical Western diets containing seed oils.
Although I’ve demonstrated that the claim that the consumption of linoleic-acid-rich seed oils increases inflammatory blood markers doesn’t hold up, and that the evidence might even point in the other direction (a possible protective effect, relative to other fats), I doubt this will have much effect on changing the narrative of those so vehemently against the use of seed oils. However, I have one request. If you’re someone who’s open to an opinion born out of an extensive review of the literature (by way of four articles**), will you now, at least, please question anyone making the assertion that seed oils are proinflammatory and demand that they critically appraise any evidence they present. Is that too much to ask?
* Note: this paper has been retracted [28], meaning that we should approach the data with caution. Surprisingly, there is little data on the LA to AA conversion in vivo. Nevertheless, I still decided to use this conversion factor in order to illustrate my point. Any challenges on this decision, please refer to the epidemiology.
** So far; I’m already working on The Exoneration of Seed Oils Part 4!
References:
1. Collier, J. (2023) ‘The Omega Ratio’, Thought for Food, 17 July. Available at: https://jamescollier.substack.com/p/the-omega-ratio-useful-or-an-unnecessary (Accessed: 8 July 2024).
2. Collier, J. (2023) ‘The Exoneration of Seed Oils’, Thought for Food, 20 July. Available at: https://jamescollier.substack.com/p/the-exoneration-of-seed-oils (Accessed: 8 July 2024).
3. Collier, J. (2024) ‘The Exoneration of Seed Oils Part 2’, Thought for Food, 12 March. Available at: https://jamescollier.substack.com/p/the-exoneration-of-seed-oils-part (Accessed: 8 July 2024).
4. (a) Wellen, K. E. and Hotamisligil, G. S. (2003) ‘Obesity-Induced Inflammatory Changes in Adipose Tissue’, Journal of Clinical Investigation, 112(12), 1785-8; (b) Dantzer, R. (2012) ‘Depression and Inflammation: An Intricate Relationship’, Biological Psychiatry, 71(1), 4-5; (c) Dantzer, R. et al. (2008) ‘From Inflammation to Sickness and Depression: When the Immune System Subjugates the Brain’, Nature Reviews. Neuroscience, 9(1), 46-56; (d) Parkitny, L. et al. (2013) ‘Inflammation in Complex Regional Pain Syndrome: A Systematic Review and Meta-Analysis’, Neurology, 80(1), 106-17; (e) Minihane, A. M. et al. (2015) ‘Low-Grade Inflammation, Diet Composition and Health: Current Research Evidence and Its Translation’, British Journal of Nutrition, 114(7), 999-1012.
5. (a) ibid (4e); (b) Hotamisligil, G. S. (2006) ‘Inflammation and Metabolic Disorders’, Nature, 444(7121), 860-7.6. (a) Calder, P. C. (2009) ‘Polyunsaturated Fatty Acids and Inflammatory Processes: New Twists in an Old Tale’, Biochimie, 91(6), 791-5; (b) Virtanen, J. K. et al. (2018) ‘The Associations of Serum n-6 Polyunsaturated Fatty Acids with Serum C-Reactive Protein in Men: The Kuopio Ischaemic Heart Disease Risk Factor Study’, European Journal of Clinical Nutrition, 72(3), 342-8; (c) University of Eastern Finland (2017) ‘Omega-6 Fatty Acids Do Not Promote Low-Grade Inflammation’, Science Daily, 13 November. Available at: https://www.sciencedaily.com/releases/2017/11/171113095430.htm (Accessed: 8 July 2024).
7. (a) Jump, D. B. (2002) ‘The Biochemistry of n-3 Polyunsaturated Fatty Acids’, Journal of Biological Chemistry, 277(11), 8755-8; (b) Flock, M. R. et al. (2013) ‘Long-Chain Omega-3 Fatty Acids: Time to Establish a Dietary Reference Intake’, Nutrition Reviews, 71(10), 692-707.
8. (a) ibid (6a); (b) ibid (6c).
9. (a) ibid (7a); (b) ibid (6a).
10. (a) Bannenberg, G. and Serhan, C. N. (2010) ‘Specialized Pro-Resolving Lipid Mediators in the Inflammatory Response: An Update’, Biochimica et Biophysica Acta, 1801(12), 1260-73; (b) Serhan, C. N. and Chiang, N. (2013) ‘Resolution Phase Lipid Mediators of Inflammation: Agonists of Resolution’, Current Opinion in Pharmacology, 13(4), 632-40.
11. (a) Calder, P. C. (2013) ‘n-3 Fatty Acids, Inflammation and Immunity: New Mechanisms to Explain Old Actions’, The Proceedings of the Nutrition Society, 72(3), 326-36; (b) Nicolaou, A. et al. (2014) ‘Polyunsaturated Fatty Acid-Derived Lipid Mediators and T Cell Function’, Frontiers in Immunology, 5, 75.
12. (a) ibid (6b); (b) ibid (6c).
13. (a) Thies, F. et al. (2001) ‘Influence of Dietary Supplementation with Long-Chain n-3 or n-6 Polyunsaturated Fatty Acids on Blood Inflammatory Cell Populations and Functions and on Plasma Soluble Adhesion Molecules in Healthy Adults’, Lipids, 36(11), 1183-93; (b) Kelley, D. S. et al. (1998) ‘Arachidonic Acid Supplementation Enhances Synthesis of Eicosanoids Without Suppressing Immune Functions in Young Healthy Men’, Lipids, 33(2), 125-30; (c) Rett, B. S. and Whelan, J. (2011) ‘Increasing Dietary Linoleic Acid Does Not Increase Tissue Arachidonic Acid Content in Adults Consuming Western-Type Diets: A Systematic Review’, Nutrition & Metabolism, 8, 36; (d) Kaikkonen, J. E. et al. (2014) ‘High Serum n6 Fatty Acid Proportion Is Associated with Lowered LDL Oxidation and Inflammation: The Cardiovascular Risk in Young Finns Study’, Free Radical Research, 48(4), 420-6.
14. Liou, Y. A. and Innis, S. M. (2009) ‘Dietary Linoleic Acid Has No Effect on Arachidonic Acid, but Increases n-6 Eicosadienoic Acid, and Lowers Dihomo-γ-Linolenic and Eicosapentaenoic Acid in Plasma of Adult Men’, Prostaglandins, Leukotrienes and Essential Fatty Acids, 80(4), 201-6.
15. Wang, B. et al. (2021) ‘Metabolism Pathways of Arachidonic Acids: Mechanisms and Potential Therapeutic Targets’, Signal Transduction and Targeted Therapy, 6, 94.
16. Reinders, I. et al. (2012) ‘Association of Serum n-3 Polyunsaturated Fatty Acids with C-Reactive Protein in Men’, European Journal of Clinical Nutrition, 66(6), 736-41.
17. (a) Simopoulos, A. P. (2008) ‘The Omega-6/Omega-3 Fatty Acid Ratio, Genetic Variation, and Cardiovascular Disease’, Asia Pacific Journal of Clinical Nutrition, 17(S1), 131-4; (b) Julia, C. et al. (2013) ‘Dietary Patterns and Risk of Elevated C-Reactive Protein Concentrations 12 Years Later’, British Journal of Nutrition, 110(4), 747-54.
18. Simopoulos, A. P. (2006) ‘Omega-6/Omega-3 Essential Fatty Acid Ratio and Chronic Disease’, Food Reviews International, 20(1), 77-90.
19. Hussein, N. (2005) ‘Long-Chain Conversion of [13C]Linoleic Acid and α-Linolenic Acid in Response to Marked Changes in Their Dietary Intake in Men’, Journal of Lipid Research, 46(2), 269-80.
20. Masson, C. J. and Mensink, R. P. (2011) ‘Exchanging Saturated Fatty Acids for (n-6) Polyunsaturated Fatty Acids in a Mixed Meal May Decrease Postprandial Lipemia and Markers of Inflammation and Endothelial Activity in Overweight Men’, Journal of Nutrition, 141(5), 816-21.
21. Nicol, K. et al. (2022) ‘No Evidence of Differential Impact of Sunflower and Rapeseed Oil on Biomarkers of Coronary Artery Disease or Chronic Kidney Disease in Healthy Adults with Overweight and Obesity: Result from a Randomised Control Trial’, European Journal of Nutrition, 61(6), 3119-33.
22. Bjermo, H. et al. (2012) ‘Effects of n-6 PUFAs Compared with SFAs on Liver Fat, Lipoproteins, and Inflammation in Abdominal Obesity: A Randomized Controlled Trial’, American Journal of Clinical Nutrition, 95(5), 1003-12.
23. Esmaillzadeh, A. and Azadbakht, L. (2008) ‘Home Use of Vegetable Oils, Markers of Systemic Inflammation, and Endothelial Dysfunction Among Women’, American Journal of Clinical Nutrition, 88(4), 913-21.
24. Telle-Hansen, V. H. et al. (2017) ‘Does Dietary Fat Affect Inflammatory Markers in Overweight and Obese Individuals? – A Review of Randomized Controlled Trials from 2010 to 2016’, Genes & Nutrition, 12, 26.
25. Johnson, G. H. and Fritsche, K. (2012) ‘Effect of Dietary Linoleic Acid on Markers of Inflammation in Healthy Persons: A Systematic Review of Randomized Controlled Trials’, Journal of the Academy of Nutrition and Dietetics, 112(7), 1029-41.
26. Higdon, J. et al. (2019) Essential fatty acids. Linus Pauling Institute. Available at: https://lpi.oregonstate.edu/mic/other-nutrients/essential-fatty-acids (Accessed: 8 July 2024).
27. Gao, F. et al. (2010) ‘Quantifying Conversion of Linoleic to Arachidonic and Other n-6 Polyunsaturated Fatty Acids in Unanesthetized Rats’, Journal of Lipid Research, 51(10), 2940-6.
28. 'Erratum' (2014) Journal of Lipid Research, 55(5), 991, https://doi.org/10.1194/jlr.M005595ERR
29. Ameur, A. et al. (2012) ‘Genetic Adaptation of Fatty-Acid Metabolism: A Human-Specific Haplotype Increasing the Biosynthesis of Long-Chain Omega-3 and Omega-6 Fatty Acids’, American Journal of Human Genetics, 90(5), 809-20.
30. (a) Adam, O. et al. (2003) ‘Influence of Dietary Linoleic Acid Intake with Different Fat Intakes on Arachidonic Acid Concentrations in Plasma and Platelet Lipids and Eicosanoid Biosynthesis in Female Volunteers’, Annals of Nutrition and Metabolism, 47(1), 31-6; (b) ibid (14).
31. ibid (13c).
32. Valsta, L. M. et al. (1996) ‘Alpha-Linolenic Acid in Rapeseed Oil Partly Compensates for the Effect of Fish Restriction on Plasma Long Chain n-3 Fatty Acids’, European Journal of Clinical Nutrition, 50(4), 229-35.
33. (a) Lasserre, M. et al. (1985) ‘Effects of Different Dietary Intake of Essential Fatty Acids on C20∶3ω6 and C20∶4ω6 Serum Levels in Human Adults’, Lipids, 20(4), 227-33; (b) Raatz, S. K. et al. (2001) ‘Total Fat Intake Modifies Plasma Fatty Acid Composition in Humans’, The Journal of Nutrition, 131(2), 231-4; (c) King, I. B. et al. (2006) ‘Effect of a Low-Fat Diet on Fatty Acid Composition in Red Cells, Plasma Phospholipids, and Cholesterol Esters: Investigation of a Biomarker of Total Fat Intake’, The American Journal of Clinical Nutrition, 83(2), 227-36.
34. Calder, P. C. (2007) ‘Dietary Arachidonic Acid: Harmful, Harmless or Helpful?’, British Journal of Nutrition, 98(3), 451-53.
35. (a) Seyberth, H. W. et al. (1975) ‘Increased Arachidonate in Lipids After Administration to Man: Effects on Prostaglandin Biosynthesis’, Clinical Pharmacology & Therapeutics, 18(5), 521-9; (b) ibid (7a); (c) ibid (7b).
36. (a) Kusumoto, A. et al. (2007) ‘Effects of Arachidonate-Enriched Triacylglycerol Supplementation on Serum Fatty Acids and Platelet Aggregation in Healthy Male Subjects with a Fish Diet’, The British Journal of Nutrition, 98(3), 626-35; (b) ibid (34).
37. (a) Public Health England (2018) National Diet and Nutrition Survey: Results from Years 7 and 8 (Combined) of the Rolling Programme (2014/2015 to 2015/2016). Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/699241/NDNS_results_years_7_and_8.pdf (Accessed: 24 June 2024); (b) US Department of Agriculture (2023) Food Availability (Per Capita) Data System: Loss-Adjusted Food Availability. Available at: https://www.ers.usda.gov/data-products/food-availability-per-capita-data-system/loss-adjusted-food-availability-documentation/ (Accessed: 24 June 2024).
38. ibid (26).