Is Calorie Tracking Accurate?
The Problem with Atwater in Calculating the Energy Value of Food
Why do nutritionists rarely agree? One minute, we’re told something’s good for us, and the next, we should avoid it. Take carbs, for example: experts tell us that carb-rich foods should be part of a nutritious meal, yet others claim that carbs are the Devil incarnate. Advice in the past has been to cut out eggs; now they’re good for us. Fat is bad; no it’s not. Oh, if I was given a penny each time I heard someone say, “Nutritionists are always changing their minds!” It’s a little odd, therefore, that the way we estimate the calorie values of foods has changed little over the past 120 years. The Atwater system – the widely recognised method of calculating calories – is pretty much embraced by all when we discuss the energy value of food and diets.
Research by the American 19th-century agricultural chemist Wilbur Olin Atwater has laid the groundwork for much of modern nutrition science. Pioneering a number of research areas, in 1896, he published The Chemical Composition of American Food Materials. This definitive collection of food data included the “fuel value” – i.e. calories – of all the foods analysed [1]. The tables were expanded and updated, and the 1906 edition went on to serve as the model for the USDA Agriculture Handbook – a catalogue of the nutritive value of American foods relied on by dietitians and nutritionists – until 1940 [2]. Following his death in 1907, Atwater’s legacy continued to influence the health of people the world over, and his work helped secure the term “calorie” firmly in our collective consciousness. His influence can be found on menus, diet-tracking apps and food labels. W.O. Atwater’s principal legacy – the Atwater system of calories – lives on today.
Measuring Calories
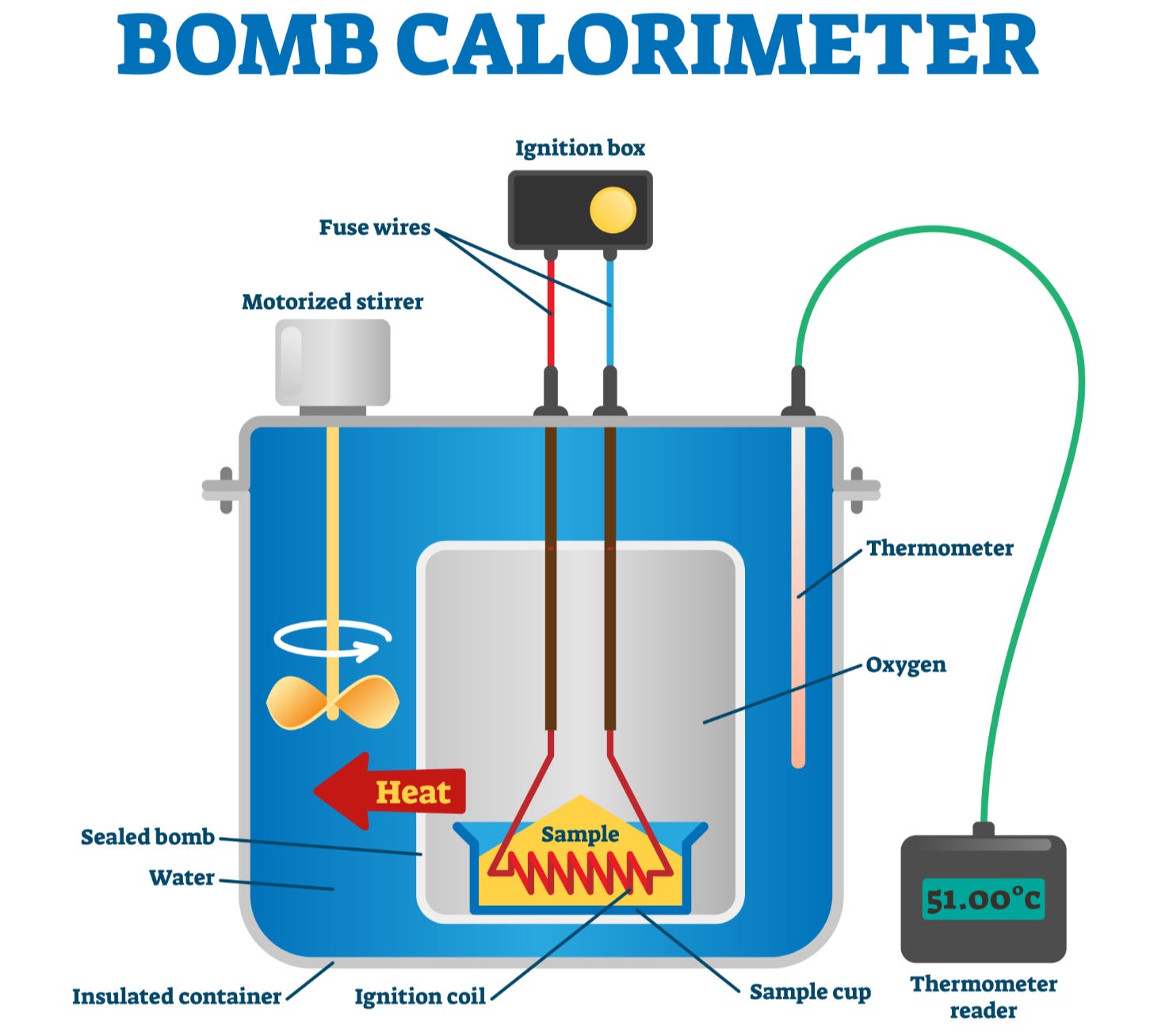
To determine the energy value of a food – i.e. its calories – a device, known as a bomb calorimeter, measures the heat of combustion of a sample of food placed in an airtight chamber filled with pure oxygen, which, in turn, is situated in a tank of water. The food is ignited by an electric spark and allowed to burn up completely. Because the bomb is sealed, no energy can escape, meaning that all the heat given off during combustion is captured by a surrounding water jacket. The resulting temperature increase in the water is measured, and from this, the energy content of the food can be calculated, since one calorie is the amount of energy needed to raise the temperature of a gram of water by one degree Celsius. Liquid foods, such as milk, fruit juice and soups are dehydrated first; as water contains no calories, removing it has no effect on the calorie content.
Bomb calorimetry is, unfortunately, rather crude when compared to the way the human body uses food, and consequently, it isn’t wholly accurate. The inside of a bomb calorimeter is a far more extreme environment than the human gastrointestinal system, where numerous other factors come into play. For example, proteins are completely burned up in the bomb calorimeter, whereas in the human body, the process of digesting proteins has a relatively large energy cost compared to other macronutrients, and proteins are not solely used for energy but also for the production of things like skin, hair, enzymes, hormones, muscle tissue and more.
Today, calorimetry is rarely used for calculating the energy content of foods. Instead, food table and label values are based on indirect estimations using the Atwater system. However, it was while using a bomb calorimeter that W.O. Atwater originally came up with his macronutrient calorie values by measuring the heats of combustion [3]. The values used today are:
Carbohydrate = 4 kcal/g
Fat = 9 kcal/g
Protein = 4 kcal/g
Fibre = 2 kcal/g
Alcohol = 7 kcal/g [4]
To illustrate, let’s use an example. The calories of a snack with 20 g of carbs, 2 g of fat, 10 g of protein and 2.5 g of fibre would be calculated as follows:
(20 g x 4 kcal) + (2 g x 9 kcal) + (10 g x 4 kcal) + (2.5 g x 2 kcal) =
80 kcal + 18 kcal + 40 kcal + 5 kcal = 143 kcal
Add a small glass of wine containing 14 g of alcohol and you’d add another (14 g x 7 kcal) 98 kcal, giving a total of 241 kcal.
A degree of inaccuracy is acknowledged with the Atwater factors when compared to bomb calorimetry, which itself isn’t entirely accurate. Indeed, the energy values for macronutrients are not as clear as the Atwater system implies. Far from it. Our food also consists of other substances that provide energy – for instance, polyols and organic acids. Moreover, “carbohydrate”, “fat” and “protein” are merely umbrella classifications, each encompassing a range of compounds, and, while the majority of these provide nutrition, the actual calorie contribution from each varies considerably. This means that there’s significant variation between what calculating through the standard methodology tells us and the calories from food that actually goes into nourishing us.
Carb Calorie Caution
Of all the macronutrients, carbs are the most problematic when seeking accuracy in calorie values. To start with, the conversion factor doesn’t distinguish between sugars, starch and fibre. Monosaccharides (such as glucose and fructose) have combustion heats of around 3.75 kcal/g, disaccharides (like sucrose (table sugar) and lactose (milk sugar) combust at approximately 3.95 kcal/g, and starch at 4.15 to 4.20 kcal/g [5]. Dietary fibre is the catch-all term for carbs that the enzymes of the human digestive system can’t break down. When we talk about fibre, we’re referring to all the long-chain carbohydrates in food other than starch, hence why some refer to fibre as “non-starch polysaccharide” (NSP). When labs analyse the chemical composition of foods, analysis methods differ, are prone to error and sometimes capture digestible starch and sugars instead of non-digestible fibre and vice versa. This means that, because the fibre and available carbohydrate values are unreliable, the resulting calories can be incorrect, as digestible carbs calculations use the 4 kcal/g value whereas fibre uses 2 kcal/g. If the calculations are done correctly – which isn’t always the case – the fibre value should be subtracted from the total carbohydrate figure before the calories are totalled.
Our gut microbiome (the bacteria, fungi, archaea and viruses that live symbiotically with us in our intestines) helps us break down the fibre in our food to short-chain fatty acids, which, in turn, can be absorbed and used for energy. Many types of fibre are partially degraded in the large intestine, where much of it is fermented, going on to provide metabolisable energy. This is why the fibre we consume has a caloric value assigned to it. However, the 2 kcal figure is an estimation, and the extent of degradation depends on both the individual and the source of fibre. Different fibres vary in their capacity to ferment, and our microbiome varies between individuals and in the same person at different times. There is no clear data on the true energy provision of fibre energy: 2 kcal/g is merely a close approximation, albeit considerably imprecise.
Polyols are organic compounds used in food production to provide sweetness or for functional reasons. Common examples include sorbitol, erythritol and glycerol. Their assigned calorie value is 2.4 kcal/g [6]. A product with a legally compliant label will have used this value when factoring the polyol contribution to its calories. However, in some cases, a brand may include polyols as part of the total carbs value, in which case 4 kcal/g may have been used in the calculation. Energy calculations in polyol-containing foods and beverages, therefore, vary considerably, often overestimating the actual calories supplied. Things are more unreliable still because the 2.4 kcal value is merely an approximation of different polyol compounds each having significantly different combustion values. For example, the common sweeteners sorbitol and xylitol combust at between 2.4 and 2.6 kcal/g, and 2.4 and 3.0 kcal/g respectively, depending on which reference you use. Others: mannitol is 1.6 kcal/g, lactitol is 2.4 kcal/g, maltitol is 3.0 kcal/g and glycerol is 4.3 kcal/g [7]. Erythritol – a sweetener commonly used in low-calorie products – is the exception, calculated separately using zero calories even though it combusts at 0.2 kcal/g. Other carbohydrate compounds can be lumped in with either total carbohydrates or fibre. Isomaltulose (also known as Palatinose – a low glycaemic sweet-tasting carbohydrate) supplies 2.0 kcal/g, trehalulose (used to prolong the shelf-life of foods) provides 3.6 kcal/g and partially hydrolysed starch yields anything from 2 to 4 kcal/g, depending on the process [8]. Aside from erythritol, the true caloric contribution of polyols and other non-starch carbs varies from 1.6 to 4.3 kcal/g – quite a range! Of course, food manufacturers and legislators need to keep things simple, and it makes sense to have a standardised single value, but the fact that we rely on approximations acknowledges the unreliability of calorie calculations.
These inaccuracies were demonstrated in a 2019 paper, which looked at the heats of combustion of six carbohydrates (glucose, fructose, sucrose, maltose, starch and cellulose) in 68 foods and proposed calorie values for fruits (3.88 kcal/g), vegetables (3.98 kcal/g) and cereals (4.13 kcal/g), noting that these figures were themselves merely estimations based on the results, demonstrating that “the use of the Atwater’s value involves a clear overestimation of the heat of combustion of the carbohydrate mass contained in vegetable source foods” [9].
The contribution that carbs make to the calories on food labels also depends on where you live. In the US, the “carbohydrate by subtraction” method is used and the resulting “total carbohydrate” value contains sugars, starch, fibre and polyols (except erythritol), i.e. the value represents Atwater’s 4 kcal/g for all carbohydrates including fibre. For nutrition labelling in the EU, carbohydrate is defined as “available carbohydrate”, which does not include the fibre component and is, instead, derived by calculating the sum of sugars and starches in the food. Fibre is calculated separately using the 2 kcal/g figure [10]. “Net carbs” – a term you might see on food labels of US-derived products – means essentially the same as available carbs. The inconsistency of what counts as carbohydrate makes the reliability of the calorie value of a food highly questionable.
Protein Prudence
Similarly, the energy provided by the protein from food is significantly misrepresented in a few respects. The first relates to amino acids. Proteins are made up of chains of amino acids wrapped around each other in complex fashions and held together by bonds. The heats of combustion of individual amino acids are different [11], meaning that the protein conversion factor of 4 kcal/g, the average used for all amino acids [12], might throw off the actual calorie contribution of a protein-rich food considerably. If, say, a food contains a large amount of phenylalanine, which yields a relatively high 6.7 kcal/g [13], the true calories provided by the food might be higher than estimated. The table below shows the heats of combustion of the 20 main amino acids: note the considerable variation from the routinely used 4 kcal/g factor [14].
The second misrepresentation in respect of the calories from protein in food comes from the way the protein content is calculated. In food labelling, legislation mandates that a food’s protein content is calculated using the Kjeldahl method, named after the Danish chemist Johan Kjeldahl, who devised the technique in 1883 [15]. The formula is:
Protein = total Kjeldahl nitrogen (N) x 6.25
As nitrogen makes up roughly 16 percent of most proteins, the N x 6.25 factor is commonly used for calculating the protein content of foods. However, as it turns out, the 6.25 estimation isn’t all that reliable: it’s an overestimation for many protein sources but an underestimation for dairy. More accurate nitrogen conversion factors include soy at a disputed 5.71, shrimp and fish at 5.6 and cereal products at 5.4 [16]. For dairy products, the European Food Standard Authority (EFSA) has ruled that, when indicating a product’s potential to supply amino acids, the nitrogen conversion factor of N x 6.38 should be used [17]. This has led to much confusion about which figure should be used. Moreover, as there are also other methods of estimating protein, it’s highly unlikely that the stated protein in a food is correct. When you can’t be sure that the stated protein level of a product is what it actually supplies, how can you be sure that its calorie contribution is accurate? Those who follow a plant-based diet and avoid meat and dairy products are particularly prone to overestimate their protein and, consequently, their calorie intake by a significant margin.
Another issue involves something known as “protein spiking”, defined by the European Specialist Sports Nutrition Alliance (ESSNA) as “the addition of free-form amino acids and other nitrogen-rich/containing nutrients added for the primary purpose of increasing the calculated protein content of a food” [18]. This is where companies put less protein into their supplements than is listed on the label and make up the shortfall with cheaper free-form amino acids. ESSNA claims that these tactics contravene EU food law and that companies do this in order to save money [19]. Protein spiking is also a hot topic in the US, with a number of class action lawsuits being filed [20]. As the Kjeldahl method tests for nitrogen, lab test results reflect all nitrogen-containing compounds in a sample, giving no indication of the source. Thus, the protein value reflects all ingredients that contain nitrogen, including free-form amino acids, performance boosters like creatine and taurine, and additives like nitrates. The calorie value of a legally compliant supplement will have been calculated from the protein value as listed on the label, representing only the food-derived proteins in the product. Those that contain popular components like branch-chain amino acids (BCAAs), creatine and taurine, in reality, supply considerably more calories than noted on the label.
To illustrate this, let’s look at two popular supplements. As a BCAA supplement only contains free-form amino acids, a 5 g serving couldn’t claim to provide any protein. It would, therefore, be assigned a zero-calorie label value. However, as BCAAs yield energy, using the 4 kcal/g Atwater calculation, 5 g of BCAA powder would provide 20 calories. But even this wouldn’t reflect the true calories provided. A typical BCAA powder consisting of leucine, isoleucine and valine in a 2:1:1 ratio* would actually provide nearly 32 kcal ((6.524 kcal x 2.5 g) + (6.523 kcal x 1.25 g) + (5.963 kcal x 1.25 g)). A bodybuilder who necks two BCAA servings daily will likely be underestimating his calories by a significant 64 kcal. Similarly, the popular supplement creatine monohydrate is labelled to have zero calories. But the true energy supplied from a 5 g serving could be as much as 21 calories.
While the inaccuracies of protein spiking are unlikely to affect dieters who limit calories to lose weight, the issue is something to consider for many supplement-taking, calorie-tracking fitness enthusiasts seeking accuracy in their quest to maximise performance.
Faking Fats
The misrepresentation of calories extends to the fat from our food, too. Fats are made up of triglycerides, each consisting of three fatty acids and glycerol. The fat we eat consists of a large number of fatty acids, each differing in its heat of combustion, although, unlike amino acids, the differences are small. Long-chain triglycerides (LCTs) combust at around 9 kcal/g so, as LCTs are the predominant dietary fats, the food industry uses this value as the conversion factor for all fat in food. There are, however, other fats present in our diets, most notably medium-chain triglycerides (MCTs), which combust at around 8.3 kcal/g [21]. MCTs are found in several plant oils including coconut oil, where over 60 percent of the fats are MCTs. Due to its culinary versatility, coconut oil is a common feature of many household kitchens and is favoured by many chefs. As the 9 kcal/g conversion factor is used to calculate the contribution from fats, products with MCTs will not have a calorie value that is correctly representative of the energy they provide.
Lab testing methodology also contributes to discrepancies in the amount of fat that’s claimed to be present in a food with consequences for calorie calculation. Techniques used will differ depending on whether the researcher seeks to evaluate the amount of specific types of fat – like omega-3s or saturates – or if she’s only interested in the total fat present in the sample. This difference in procedure will lead to discrepancies in the way fat is determined. When testing for a type of fat, the fatty acid profile is analysed, and the result accounts for the glycerol content of the triglycerides, with the value then expressed on a sample basis to account for the fat content. The reported fatty acid data is simply a normalised percentage of the total fatty acids measured, and it does not include any conversion factor that accounts for the glycerol. This discrepancy can be corrected by multiplying each value by 0.956.
Let’s use an example. A straightforward measurement reports the total fat content of a sample to be 12.13 g. However, testing for the fatty acid profile could reveal total monounsaturates to be 5.91 g, omega-3s to be 3.52 g, omega-6s to be 0.75 g and saturates to be 2.51 g. The four values total 12.69 g – how can we have arrived at a number that’s greater than 12.13 g? By multiplying the fatty acid total by 0.956, the adjusted value becomes 12.13 g. Crucially, therefore, how the fat content is reported affects the way the calories are calculated. This differs depending on the methodology and is something consumers won’t know, casting doubt over the accuracy of the fat-derived calories claimed to be in a product.
Alcohol Anomalies
Next, let’s look at the validity of calories coming from alcoholic drinks. During fermentation, the simple sugars in the raw ingredients break down mostly to ethanol: by far the most abundant alcohol present in the drinks we consume. However, glucose also breaks down into other alcohols. Consequently, all alcoholic drinks contain a small percentage of other alcohol compounds known as congeners. It’s congeners that contribute to the distinct tastes of different distilled beverages. There are hundreds of different congeners in our drinks: whisky, for example, contains roughly 400 different alcohols. Brandy, rum and red wine have the highest amount of congeners, and vodka and beer have the least. The heat of combustion of ethanol is 7.056 kcal/g. Despite the variation born through the congeners in a drink’s make-up, the 7 kcal/g conversion factor that’s used to calculate the calories from alcohol is actually a pretty reliable estimate. However, when calculating the contribution of an alcoholic drink to calorie intake, its alcohol percentage is rarely taken into account. Rather, a generic calorie value for a particular type of drink is taken from food tables ignoring the significant variation in the alcohol content within a drinks category. Wines, for example, typically vary from 11 to 13 percent and beer from four to six percent. The calories from the alcohol in a 150ml glass of wine could range from 114 kcal to 135 kcal, and a pint of beer from 130 kcal to 200 kcal. That’s quite a range and can lead to unreliable estimations of the calories consumed from drinks.
Alcoholic drinks aren’t the only source of ethanol in modern diets. Ethanol is also used as a constituent of some food flavourings. The way brands calculate the calories on their labels often ignore this, underestimating the calorie value of the final product. And, as well as this, foods also contain other organic acids, either naturally occurring or added for preservation or for technical reasons in food processing. The assigned calorie value for organic acids is 3 kcal/g [22], yet, again, here we have an unreliable estimation: acetic acid’s heat of combustion is 3.5 kcal/g, citric acid is 2.5 kcal/g, malic acid is 2.4 kcal/g and tartaric acid 2.0 kcal/g [23], and it’s not clear if the calorie contribution of organic acids will even have been considered.
Combusting Calories
The amount of energy supplied by macronutrients varies, methods and lab techniques of measurement vary, calorie calculations vary, the ways calories are totalled vary and reporting varies. Consequently, the standard values used to calculate calories are unreliable, and the figures we see on nutrient tables and food labels are a poor representation of the true energy value provided by the food we consume. But there’s something else, too: brands are permitted significant leeway in how they report the nutritional content of their products. Calories and nutrient values may be reported either by calculation, where the proportional nutrient contribution of each ingredient in a recipe is totalled, or from lab tests performed on the product as a whole. Legislation acknowledges that there will be inconsistencies and permits up to a 20 percent variation between the macronutrient and calorie content on the labels and from the results from lab tests** [24]. For example, a food label stating that a serving contains 200 calories could range from 160 to 240 calories and still be compliant. A bit tricky for someone who thinks they’re accurately tracking their intake! There will always be significant variation resulting from different lab techniques and calculation methods, or due to variations in the nutrient content of ingredients, particularly wholefood ingredients, grown in different regions, under different conditions, using different procedures.
I hear you: this dismantling of how calories are estimated has, in places, been quite complicated. I apologise. The key take-home point is that the true energy contribution from the food we consume is likely very different from the figures provided on labels and tables. And there’s one issue I haven’t even mentioned – something that makes calorie tracking less reliable still – the topic of caloric availability is something I’ll cover in a future article.
Whether or not calorie tracking is a useful strategy to help people achieve their fitness goals is a much debated and highly contentious topic – well beyond the scope of this article – with pros and cons and convincing arguments either way. Here, I merely wish to draw attention to the multiple imprecisions inherent in the task. For many, totting up the calories in what they eat is part of their daily regimen. If you’re one of these calorie-counting aficionados, be aware that any attempt to reliably estimate the energy value of your food will have involved numerous conflicting methods, a range of techniques and multiple rounding nuances, making exactness an impossible task. The more accurate way would be to combust your meals, but, well, then you wouldn’t have any dinner left to eat …
* This is the approximate ratio in which the three are metabolised.
** In the UK and EU, calories fall outside the scope of tolerance percentage and are instead based on an average value rounded to the nearest calorie [25]. In the US, calories can be up to 20 percent over what’s on the label; the only mention of “under” in the legislation is “Reasonable deficiencies of calories … under labeled amounts are acceptable within current good manufacturing practice” [26].
References:
1. Atwater, W. O. and Woods, C. D. (1896) The Chemical Composition of American Food Materials. Washington: Government Printing Office.
2. (a) National Agricultural Library, US Department of Agriculture (n.d.) Wilbur Olin Atwater Papers. Available at: https://www.nal.usda.gov/collections/special-collections/wilbur-olin-atwater-papers (Accessed: 20 May 2024); (b) Martin, P. (2015) ‘Olympians Owe Gold Standard to a 19th-Century Chemist’, Fox News, 7 May. Available at: https://www.foxnews.com/opinion/olympians-owe-gold-standard-to-a-19th-century-chemist (Accessed: 20 May 2024).
3. Widdowson, E. (1978) ‘Note on the Calculation of the Energy Value of Foods and of Diets’, in: A. A. Paul and D. A. Southgate (Eds) The Composition of Foods. 4th edn. London: HMSO.
4. (a) Food and Agriculture Organization of the United Nations (2003) Food Energy – Methods of Analysis and Conversion Factors. Available at: https://www.fao.org/uploads/media/FAO_2003_Food_Energy_02.pdf (Accessed: 20 May 2024); (b) ‘Regulation (EU) No. 1169/2011 of the European Parliament and of the Council on the Provision of Food Information to Consumers’ (2011) Official Journal L304, 18-63; (c) Food Drink Europe (2013) Guidance on the Provision of Food Information to Consumers, Regulation (EU) No. 1169/2011. Available at: http://www.foodlaw.rdg.ac.uk/pdf/eu-13017-FDE-FIC-Guidance.pdf (Accessed: 21 May 2024).
5. (a) ibid (4a); (b) Southgate, D. A. T. (1981) The Relationship Between Food Composition and Available Energy. Provisional Agenda Item 4.1.3, Joint FAO/WHO/UNU Expert Consultation on Energy and Protein Requirements, Rome, 5–17 October.
6. (a) ibid (4a); (b) European Association of Polyol Producers (2013) The Food Energy Value of Polyols. Available at: https://polyols-eu.org/wp-content/uploads/Food_energy_value_of_polyols.pdf (Accessed: 21 May 2024).
7. (a) Chattopadhyay, S. et al. (2014) ‘Artificial Sweeteners – A Review’, Journal of Food Science and Technology, 51(4), 611-21; (b) Rice, T. et al. (2020) ‘A Review of Polyols – Biotechnological Production, Food Applications, Regulation, Labeling and Health Effects’, Critical Reviews in Food Science and Nutrition, 60(12), 2034-51.
8. ibid (7a).
9. Martínez-Navarro, A. G. et al. (2019) ‘Heats of Combustion Representative of the Carbohydrate Mass Contained in Fruits, Vegetables, or Cereals’, Food Science & Nutrition, 7(9), 3119-27.
10. Trustwell (2015) How Carbs Are Calculated in Different Countries. Available at: https://blog.trustwell.com/how-carbs-are-calculated-in-different-countries (Accessed: 21 May 2024).
11. May, M. E. et al. (1990) ‘Energy Content of Diets of Variable Amino Acid Composition’, American Journal of Clinical Nutrition, 52(5), 770-6.
12. Sands, R. (1974) ‘Rapid Method for Calculating Energy Value of Food Components’, Food Technology, July, 29-40.
13. ibid (11).
14. ibid (11).
15. Kjeldahl, J. (1883) ‘Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern’ [New Method for the Determination of Nitrogen in Organic Substances], Zeitschrift für analytische Chemie, 22(1), 366-83.
16. (a) Krul, E. S. (2019) ‘Calculation of Nitrogen-to-Protein Conversion Factors: A Review with a Focus on Soy Protein’, Journal of the American Oil Chemists’ Society, 96(4), 339-64; (b) Hayes, M. (2020) ‘Measuring Protein Content in Food: An Overview of Methods’, Foods, 9(10), 1340.
17. Article 5(9) of ‘Council Directive 92/46/EEC Laying Down the Health Rules for the Production and Placing on the Market of Raw Milk, Heat-Treated Milk and Milk-Based Products’ (1992) Official Journal L268, 1-32.
18. European Specialist Sports Nutrition Alliance (n.d.) Protein Watch. Available at: https://www.essna.com/protein-watch/ (Accessed: 21 May 2024).
19. ESSNA claims that protein spiking contravenes General Food Law Principles, in particular Article 7(1) of ‘Regulation (EU) No. 1169/2011 on the Provision of Food Information to Consumers’ (2011) Official Journal L304, 18-63; Article 169 of ‘Treaty on the Functioning of the European Union’ (2016) Official Journal C202, 47-199; Article 8(1)(C) of ‘Regulation (EC) No. 178/2002 Laying Down the General Principles and Requirements of Food Law’ (2002) Official Journal L31, 1-24; and Articles 3 and 5(1) of ‘Regulation (EC) No. 1924/2006 on Nutrition and Health Claims Made on Foods’ (2006) Official Journal L404, 9-25.
20. Example: Parker Waichman LLP (n.d.) Protein Powder Product Makers Lawsuit. Available at: https://www.yourlawyer.com/product-liability/protein-spiking/ (Accessed: 21 May 2024).
21. Ingle, D. L. et al. (1999) ‘Dietary Energy Value of Medium-Chain Triglycerides’, Journal of Food Science, 64, 960-3.
22. ibid (4).
23. Department of the Treasury, Alcohol and Tobacco Tax and Trade Bureau (2004) Official Method – SSD:TM:401: Calories in Flavored Wines. Available at: https://www.ttb.gov/system/files?file=images/pdfs/ssd/ssdtm401calsflvwine.pdf (Accessed: 21 May 2024).
24. (a) European Commission (2012) Guidance Document for Competent Authorities for the Control of Compliance with EU Legislation on: Regulation (EU) No. 1169/2011 on the Provision of Food Information to Consumers. Available at: https://food.ec.europa.eu/system/files/2016-10/labelling_nutrition-vitamins_minerals-guidance_tolerances_1212_en.pdf (Accessed: 21 May 2024); (b) US Food & Drug Administration (2023) Code of Federal Regulations: Title 21: Food and Drugs. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=101.9 (Accessed: 21 May 2024).
25. ibid 24(a).
26. ibid 24(b).



I cannot remember the last time I learned so much from a single article.
Thorough and highly informative.